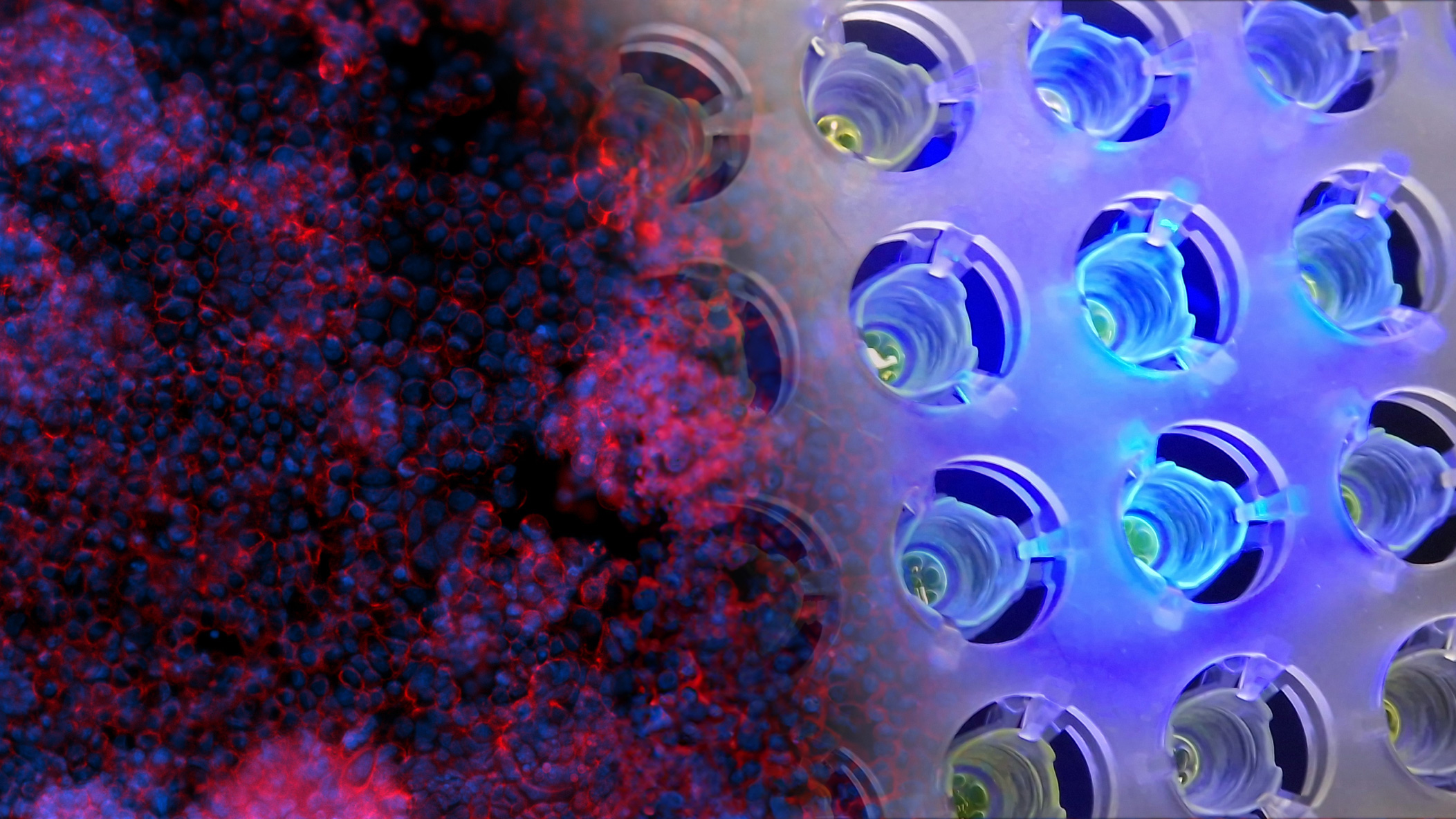
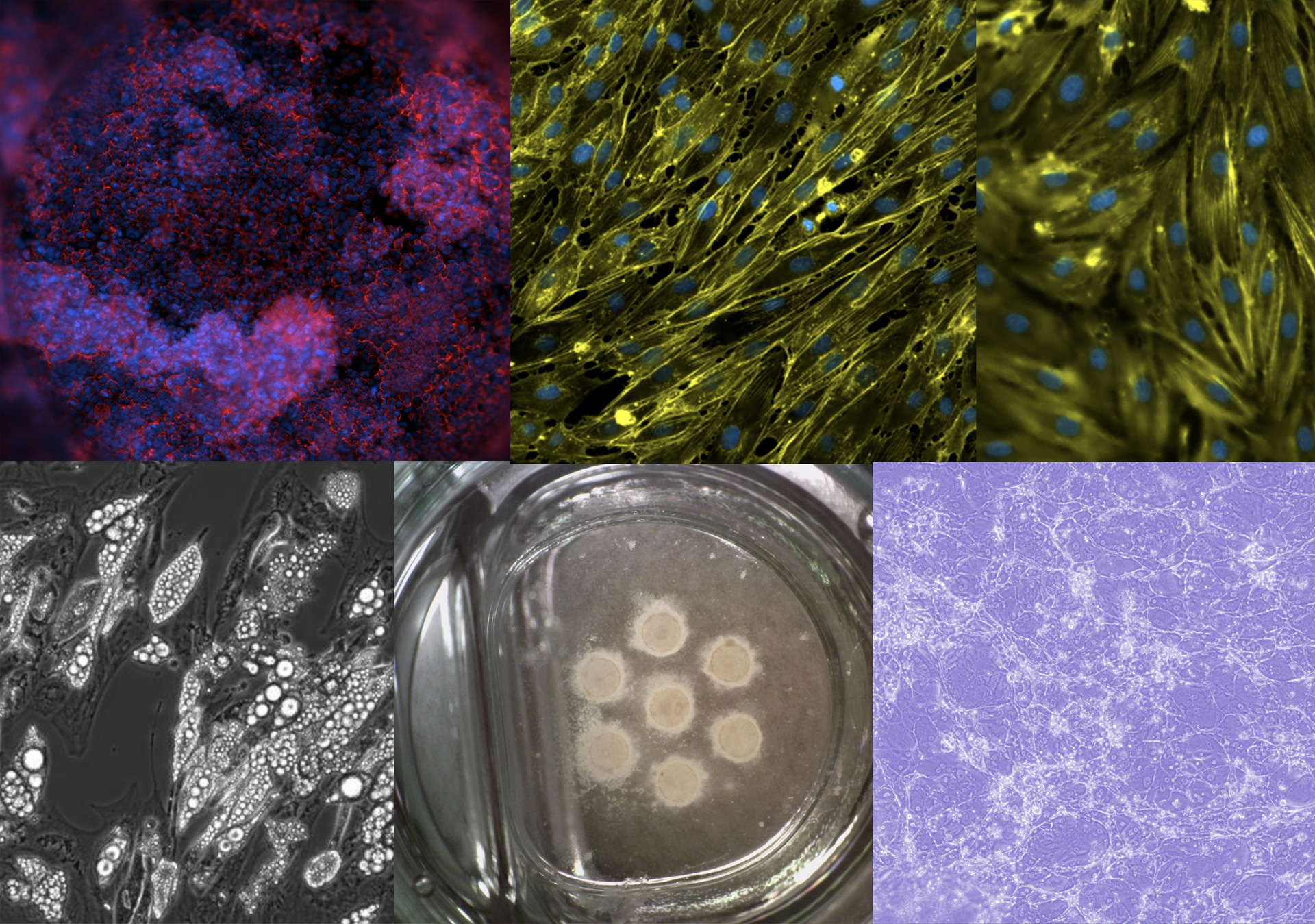
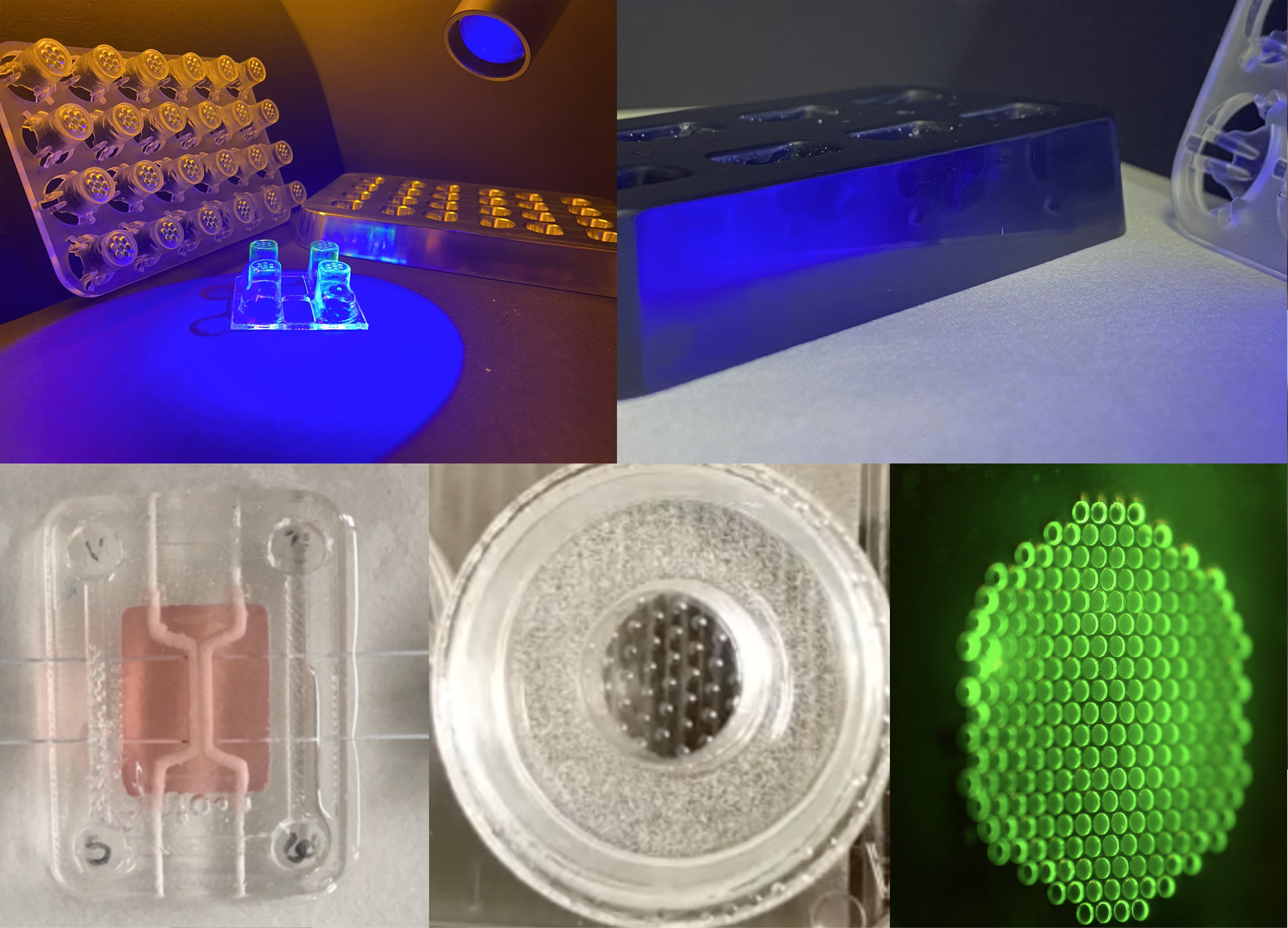
In the Scalable organ-on-a-chip system group, we aim to build advanced connected tissue models for in vitro testing of medicinal products. The application is drug development in the pharmaceutical industry and precision or personalized medicine at hospitals. However, any tests, such as toxicology, are possible applications. Our vision is to replace experimental animals with complex organ system with better predictive value. To achieve this, we apply minimal necessary engineering of organ-on-a-chip technology to obtain advanced cell culture systems that adhere to industry standards, automation, and instrumentation, and is furthermore mass producible. Through our collaborators, we are working with the intestine, liver, immune system, blood vessels, blood brain barrier, brain, fat, and cancer cells.
Some tissues are modeled using differentiation of induced pluripotent stem cells and mesenchymal stem cells while others are modelled using primary and immortalized cell lines. During prototyping, we apply 3D printing of hard materials that we subsequently modify with various hydrogels. The hydrogels are populated with cells on the inside or outside to mimic a tissue slice. Multiple of these can then be stacked on top of each other to model complex multiorgan systems, such as the gut – brain axis, for studying and interfering with virus transduction or the first pass metabolism. Shear and improved mass transfer is obtained using gravitational flow that is easily scalable.