Research Group
Colloids and Biological Interfaces (CBIO)

Group leader: Jonas Rosager Henriksen
The Colloids and Biological Interfaces (CBIO) group is a multidisciplinary research group with a mission to contribute to a better health in the society by developing healthcare technologies that improve drug delivery, immunotherapy, bioimaging, and biomedical devices. The technologies are conceptualized based on decades-long experience within nanoscale solutions for these purposes. To support this mission, the group brings together scientists from a range of diverse research fields and employs synthetic chemists, biophysicists, formulation scientists, cell biologists, immunologists, veterinarians, and medical doctors. This creates a unique scientific environment capable of handling research projects from initial concept and synthesis of therapeutic compounds and drug delivery systems to preclinical assessment as well as investigation of the biological context in which the new technology must function. The group is deeply dedicated to bringing health technologies into play in a clinical context and has provided the foundation for a series of spinout companies. Technologies of these spinout companies currently undergoes preclinical and clinical evaluation, or are marketed for use in humans.
Research projects
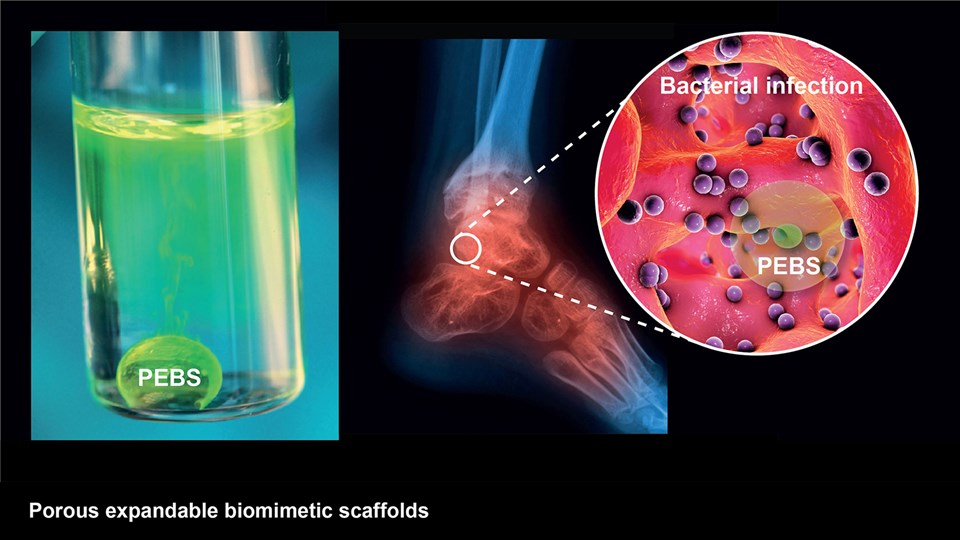
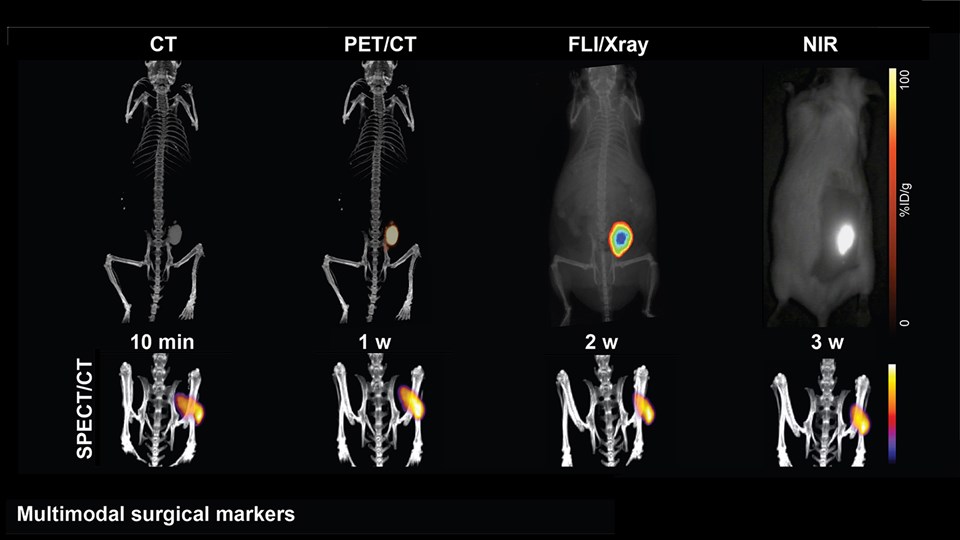
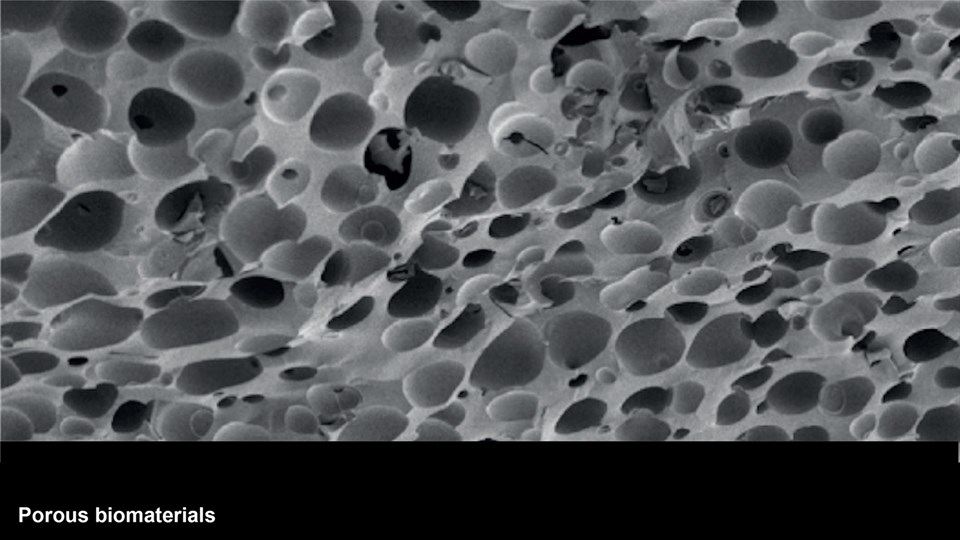
Advanced biomaterial implants for diagnostics and therapy
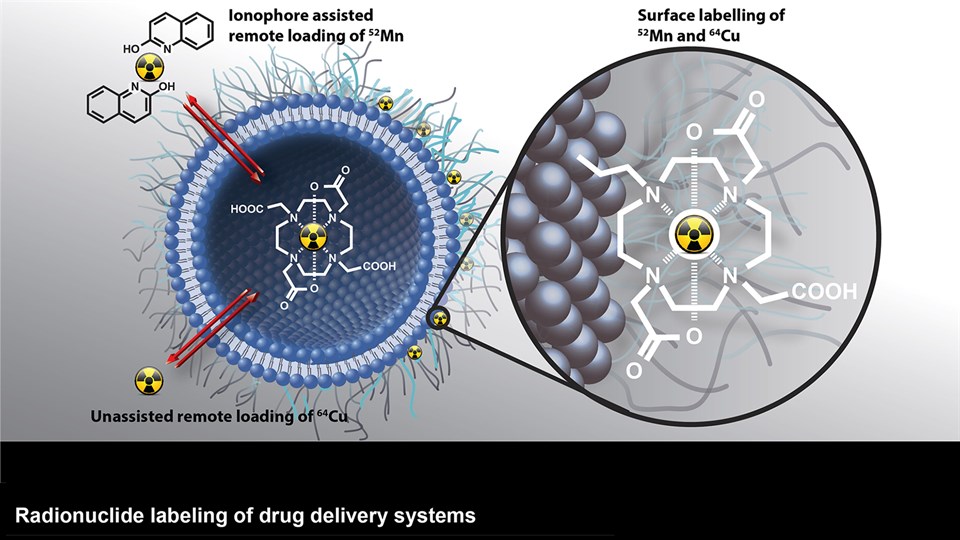
The CBIO group develops advanced biomaterials that can assist in diagnostics and therapeutic interventions. Positionally and chemically stable markers with X-ray contrast for computed tomography (CT) imaging have been developed to provide guidance during image-guided radiation therapy and proton beam therapy for cancer. These have undergone full development from bench to bedside and are now marketed for use in the clinic after trials in esophageal, bladder, and non-small cell lung cancer. Moreover, novel materials with visibility in a range of important clinical imaging modalities (PET, CT, near-infrared imaging) are being developed for surgical guidance. Projects are ongoing to create multifunctional biomaterials capable of providing mechanical support, while simultaneously releasing antibiotics or tissue growth factors in a controlled manner for application in wound healing and tissue regenerative therapy. In addition, embedding of radionuclides into advanced biomaterials are made to expand upon the potential application and efficacy of localized brachytherapy or use of these as markers in image guided and robotic surgery. One strategy to enable this is by embedding hydrophobic complexes of beta-emitters (177Lu or 90Y) to increase focal radiation dose in tumors, while limiting damage to the surrounding tissue.
Drug delivery and discovery
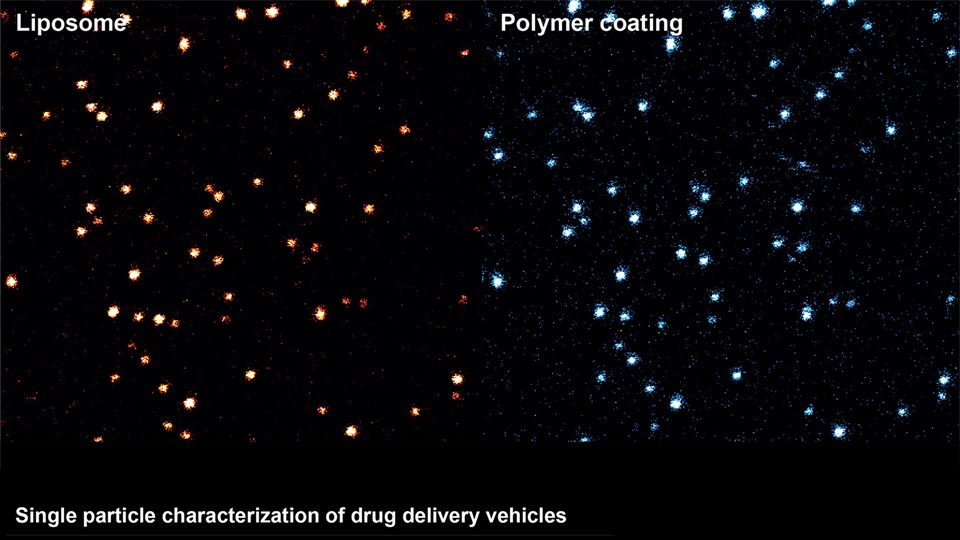
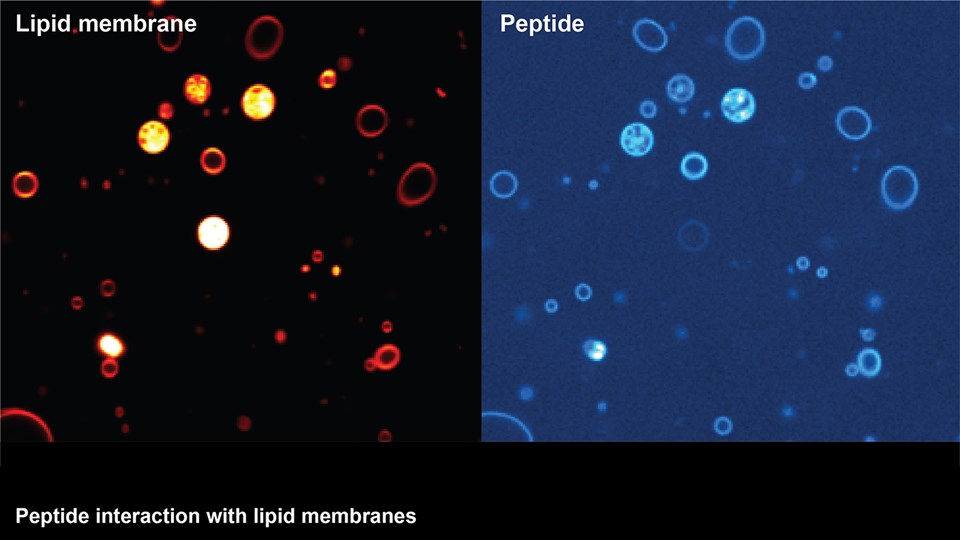
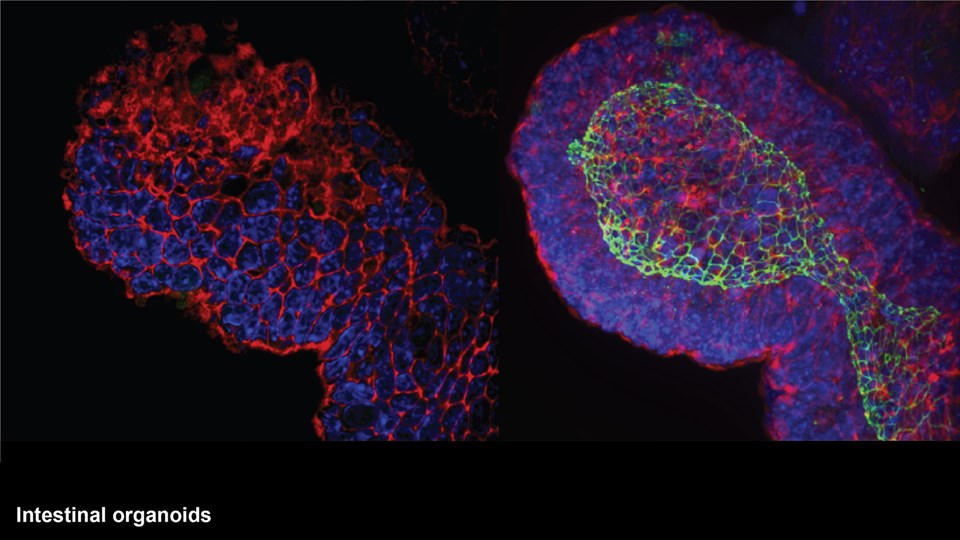
Efficient delivery of drugs to the site of disease is a crucial parameter in most therapeutic strategies. However, poor solubility and stability of drug compounds or the impenetrable character of biological barriers limits the amounts of drug that accumulates at the desired location in the body. The CBIO group runs a series of projects concerning the development of drug delivery systems capable of transporting therapeutic compounds to improve therapeutic efficacy for range of different diseases. These systems include nanoparticles and micelles comprising lipids or polymers that can encapsulate many classes of drugs, including chemotherapeutics, antibiotics, and immunostimulatory compounds. Other projects are focused on depot formulations for localized and sustained drug activity, and development of new drugs and prodrugs designed to be compatible with the different drug delivery systems. The main focus of the development projects is to improve drug delivery in cancer (see Immunotherapy and cancer vaccines), inflammatory, infectious, and brain diseases, or conditions, where oral drug delivery is paramount. With respect to brain diseases, drug delivery strategies are developed to cross the blood-brain barrier for the treatment of Alzheimer’s disease and brain tumors. Technologies include nano-sized drug carriers and antibody-based therapies. In relation to oral drug delivery, peptide transport across the intestinal epithelium is studied to inform on how large molecules can penetrate this barrier to increase drug delivery and patient compliance (see CitBIO).
Functional bioimaging and tracer development
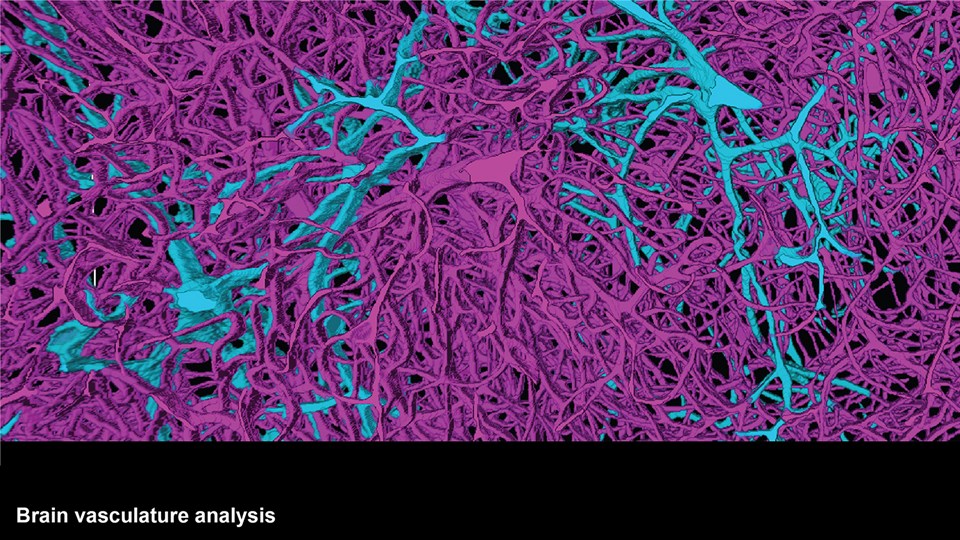
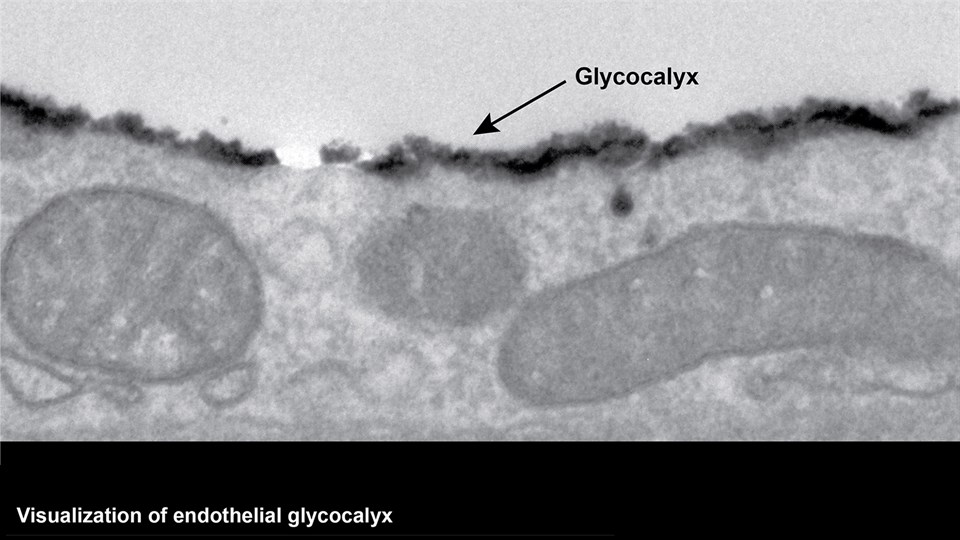
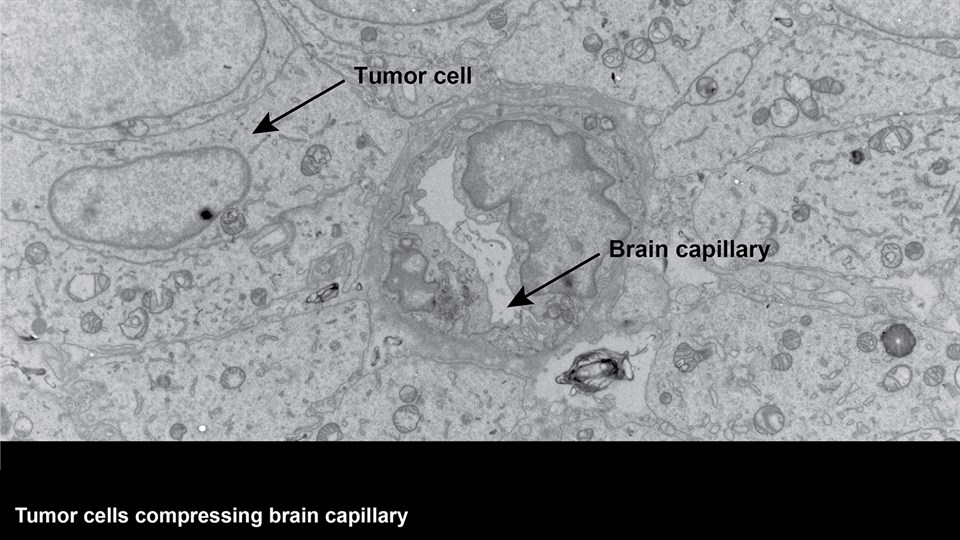
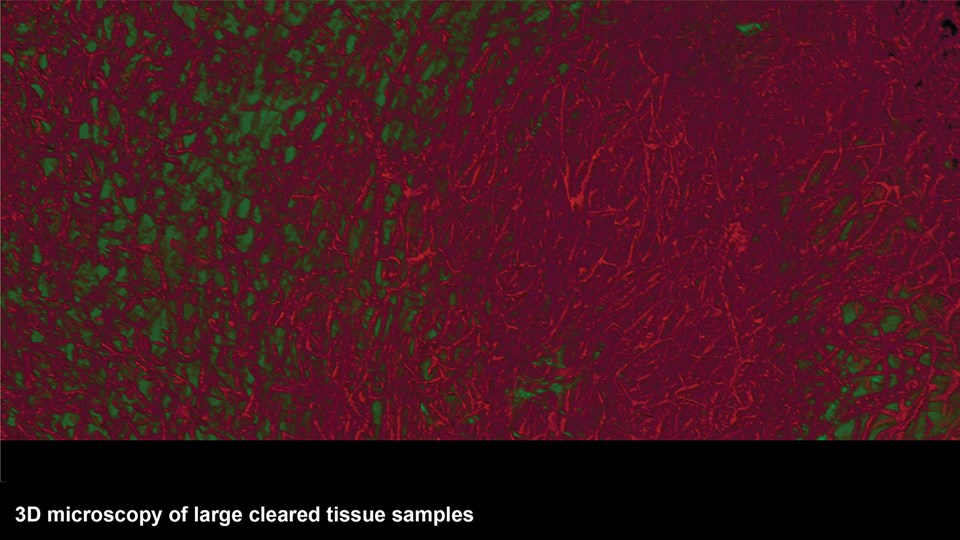
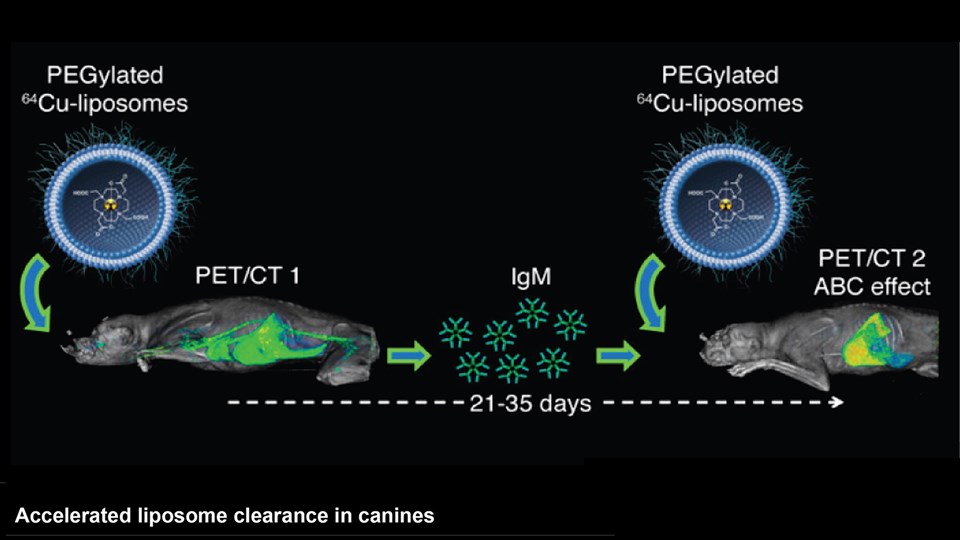
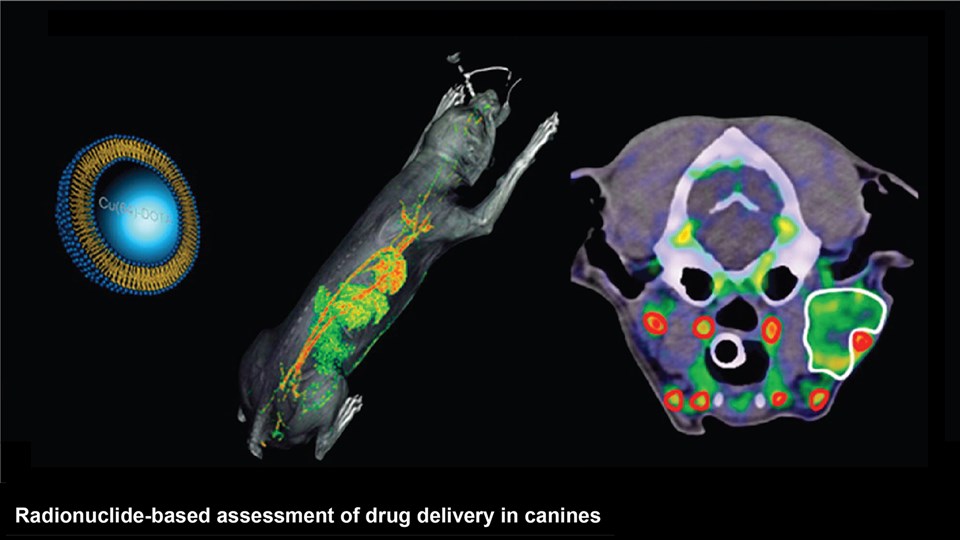
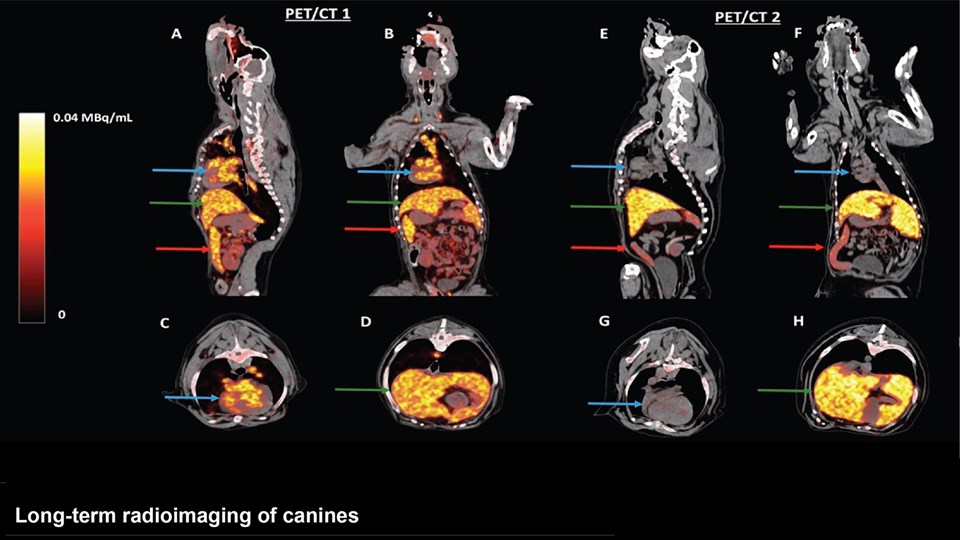
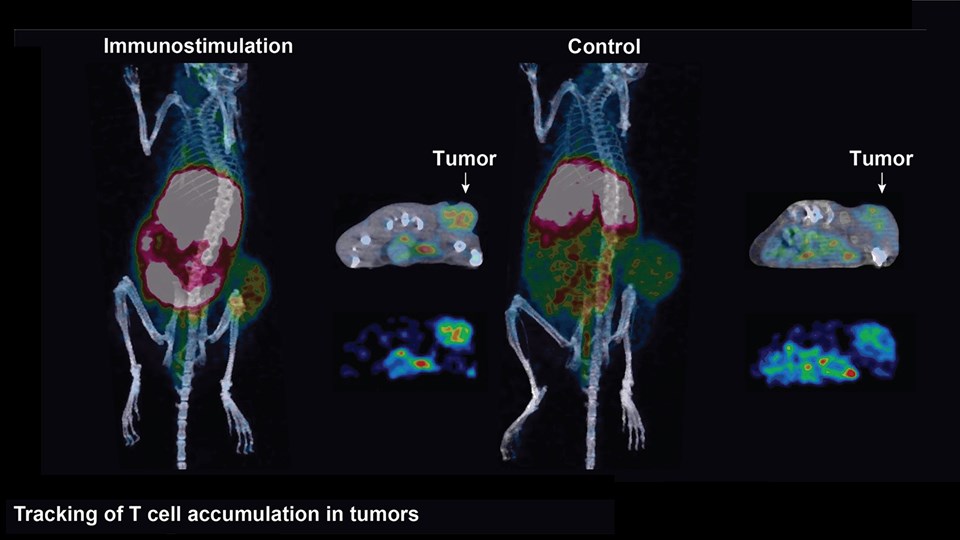
Advanced bioimaging is a central tool used to gain detailed information about drug delivery technologies and their biological performance, properties and interactions. The array of technologies available spans from small (electron and light microscopy) to larger length scales, including (i) computed tomography (CT), (ii) positron emission tomography (PET), (iii) single photon emission computed tomography (SPECT), and (iv) fluorescence in vivo imaging systems (IVIS). One of the main imaging modalities of the group is PET, which is used to evaluate pharmacokinetic behavior and biodistribution of drug delivery technologies or exogenously modified immune cells. Multiple radiolabeling methods based on novel nanomaterials has been developed to enable whole-body preclinical analysis of the drug delivery process and therapeutic response. For example, immune cells can be radiolabeled with high efficiency and tracked during the process of re-administration into the diseased subject. Radiolabeling methods have also been developed for tracing of liposomes, micelles, and various proteins (including antibodies) in small (mice and rats) and large (canines) animal models. Several advanced microscopy techniques are used in the group in connection with the development of drug delivery technologies. Spinning disk confocal and lattice light sheet microscopes are used to perform real-time imaging of drug or drug delivery system interaction with cells in culture, or to determine drug transport across cell culture models of e.g. the intestinal barrier and blood-brain barrier. Confocal microscopy is used in conjunction with optical clearing techniques to enable imaging of large 3D volumes of transparent tissue (especially brain), or for single molecule bleaching experiments that are used to perform direct quantification of therapeutic proteins, nanoparticles, or membrane receptors on cells. The activities within microscopy are closely connected to development of advanced image analysis methods, e.g. using on machine learning-based segmentation, which are used to obtain quantitative information about parameters like spatial distribution and coverage of drugs accumulation in diseased tissue or characteristics of the vascular network. Electron microscopy is used both for evaluating the ultrastructure of drug delivery systems as well as providing high resolution details of subcellular localization of drug delivery vehicles in tissues.
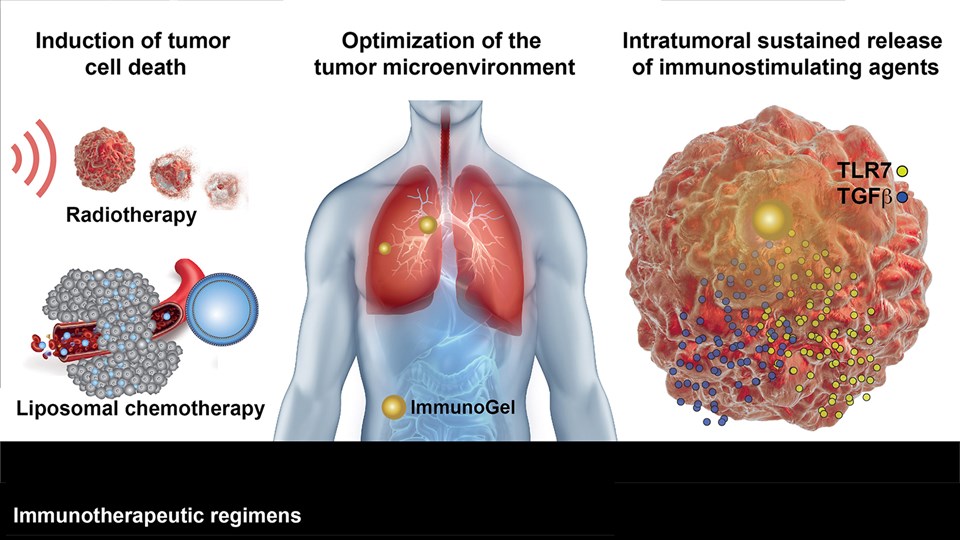
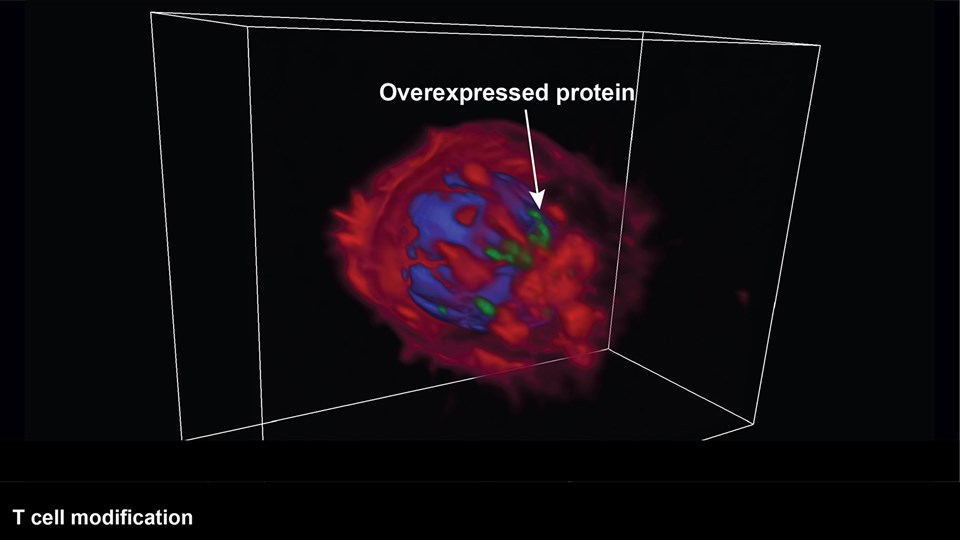
Immunotherapy and cancer vaccines
Treatment of cancer is a fundamental challenge and there is a continuous need for new inventions that are able to cure or prolong survival of patients. Fortunately, the recent years has illustrated a large potential of therapeutic success imposed by modifying the immune system in favor of cancer cell detection and destruction. The CBIO group runs a series of projects, wherein immunomodulation is induced using advanced nanotechnologies to stimulate a strong anti-cancer response. These technologies include: (i) enzyme-sensitive liposomes (lipid nanoparticles) capable of stimulating the release of tumor antigens and a downstream immune response, (ii) carbohydrate formulations enabling localized and sustained release of therapeutic compounds, pro-inflammatory cytokines, or tumor antigens, (iii) lipid micelle formulations that deliver immune-stimulating compounds to enforce anti-tumor immune responses, and (iv) various nanomaterials capable of improving the immune cell’s capacity for cancer cell destruction in adoptive cell transfer and other cell-based therapies. In addition to the expertise in chemical synthesis and formulation required to develop these technologies, the group has scientists with strong expertise in immunology as well as access to state-of-the-art instrumentation, including; flow cytometers, cell sorters, pre-clinical cancer models, and bioimaging technologies (see Functional bioimaging and tracer development). This allows for detailed studies of how the newly developed immunotherapies interact with biological systems.
Group Leader
Jonas Rosager Henriksen Associate Professor Department of Health Technology Mobile: +45 40582866 jhen@dtu.dk