Researchers from DTU Health Tech have shown how to measure the size and surface charge of nanoparticles with something as simple as table salt.
It is easy to measure the diameter of a tennis ball because we can see it with our naked eyes. But how do you measure the size of a ball with a diameter of only 70 nanometers - a million times smaller than a tennis ball - that you cannot even see in a microscope?
Researchers from DTU Health Tech have shown how to measure the size and surface charge of nanoparticles with something as simple as table salt.
These findings might have clinical application as the cells in our body secrete nanoparticles to the blood stream. PhD Student Martin Kjærulf Rasmussen explains: "Their size and surface charge depend on the cell type they came from, and therefore these nanoparticles are used for diagnostics of for example cancer or Alzheimer’s disease. So when the nanoparticles are isolated from blood or urine, they could be purified and characterized in a single step with our new method."
The measurements are performed on a plastic device with very small channels that form the letter ‘H’ (see figure). The shallow nanoslit with the shape of a funnel connects the two microchannels. A liquid with high salt concentration flows through the right microchannel, and a liquid with almost no salt flows through the left.
This establishes a salt gradient in the nanoslit that has two effects.
First, the salt gradient causes a flow from the microchannel with high salt concentration to the other due to an effect named diffusioosmosis. The fluid speed varies along the nanoslit because of the changing width. Just like a river runs faster where it narrows.
The second effect is that when nanoparticles are placed in the microchannel with low salt concentration, they spontaneously wander against the flow in the nanoslit to higher salt concentrations. This diffusiophoretic motion is driven by the salt gradient and depends on the sizes and surface charges of the nanoparticles.
The nanoslit is designed so that the nanoparticles do not pass through it. Instead they get trapped where the diffusioosmotic and diffusiophoretic effects balance. The trapping position depends on the nanoparticles’ sizes and surface charges. That is they key idea behind the method.
In the paper, the authors demonstrate that they can find the size of both individual nanoparticles, ensembles of particles, and even of particle mixtures (see figure).
The plastic device is an injection-moulded low-cost plastic chip fit for mass-production. So it might find applications as a single-use device for point-of-care tests in the health sector, in the pharmaceutical industry, or for environmental monitoring.
Read the full paper in Nature Communications here: https://www.nature.com/articles/s41467-020-15889-3
The paper was selected for Editors’ Highlights, a small number of Articles recently published in Nature Communications that the Editors believe are particularly interesting or important (see https://www.nature.com/ncomms/editorshighlights).
Figure
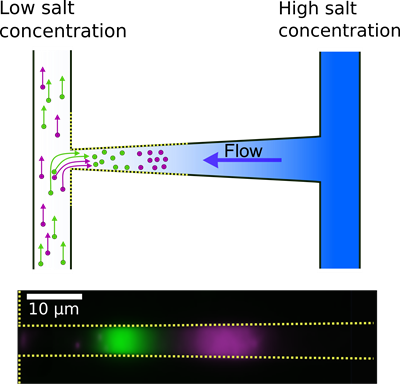
Top: A nanoslit with the shape of a funnel connects two microchannels. The salt gradient in the nanoslit pulls nanoparticles into the nanoslit and creates a flow in the opposite direction. So the particles get trapped. The trapping positions depend on the particles’ size and surface charge.
Bottom: Two populations of nanoparticles with the same diameter - but different surface charges - are trapped at different positions in the nanoslit.