Teaching in courses:
Research Group
Engineered Fluidics and Tissues

Group leader: Niels Bent Larsen
- High-resolution stereolithographic 3D printing for 3D cell culture
- Materials with engineered functions for 3D cell culture
- Materials for 3D microfluidics
- Engineered 3D microvasculature for tissue culture
- Industrially scalable 3D micromanufacturing
We explore new types and formulations of polymer materials with functions supporting tissue function, stimulation, and functional readout. Our current foci are mechanical and chemical stimulation and readout of mechanical properties of 3D tissues.
We have a particular interest in engineering 3D oxygen and nutrient supply, a grand challenge of current cultured tissues. We meet this challenge by design and 3D printed micromanufacture of massively parallel densely spaced microfluidic channel arrays made from polymers of tunable oxygen and nutrient diffusivity.
We have a key interest in making our research results applicable to societal needs. Our materials and manufacturing processes are consequently guided by a need for later industrial scalability.
Research projects
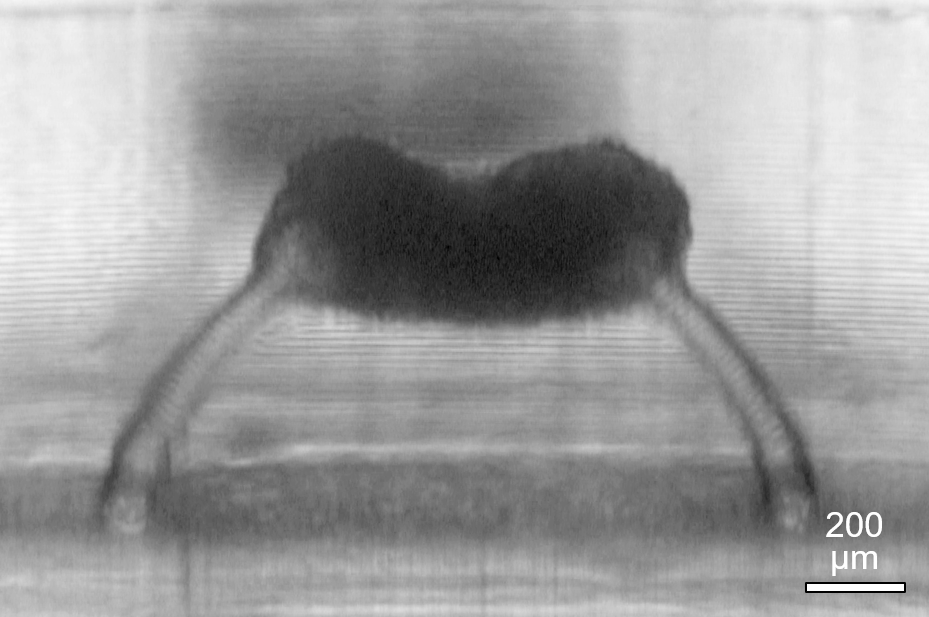
3D printed micropost supports to analyze the mechanical properties of contractile 3D tissues, in a long-term joint research effort with the company Sophion Bioscience.
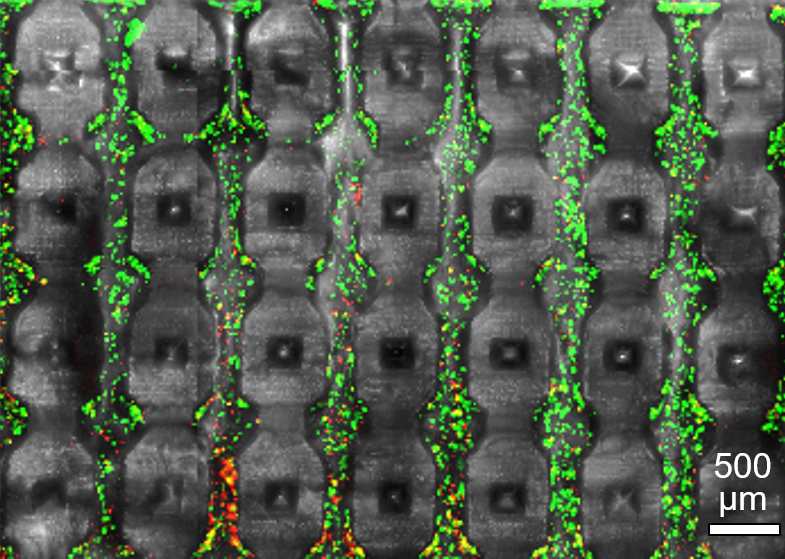
An early design generation of our 3D printed massively perfused microfluidic channel networks (here shown after mechanical cross-sectioning) supporting 3D culture at high cell densities (green = live cells).
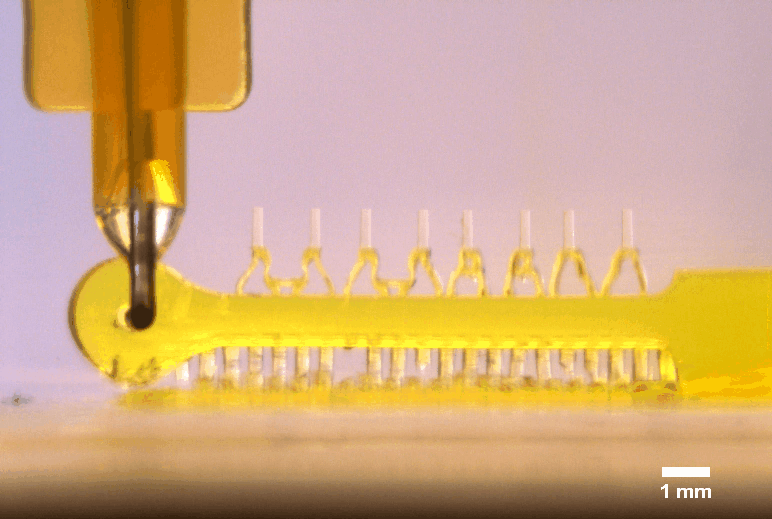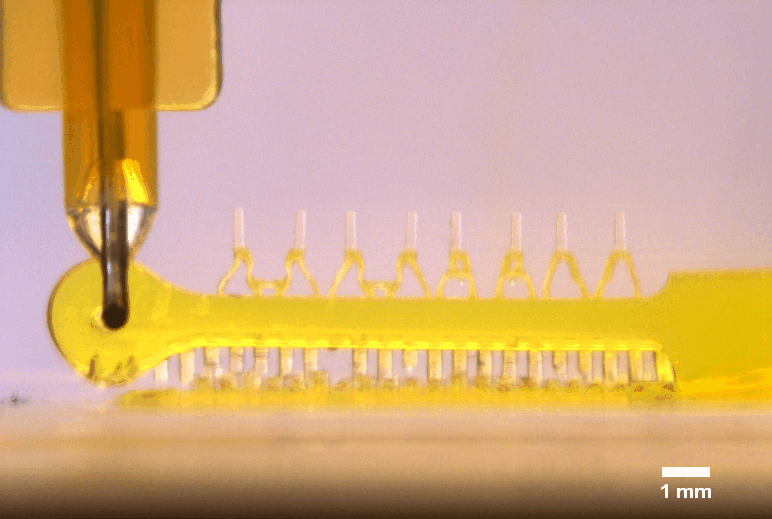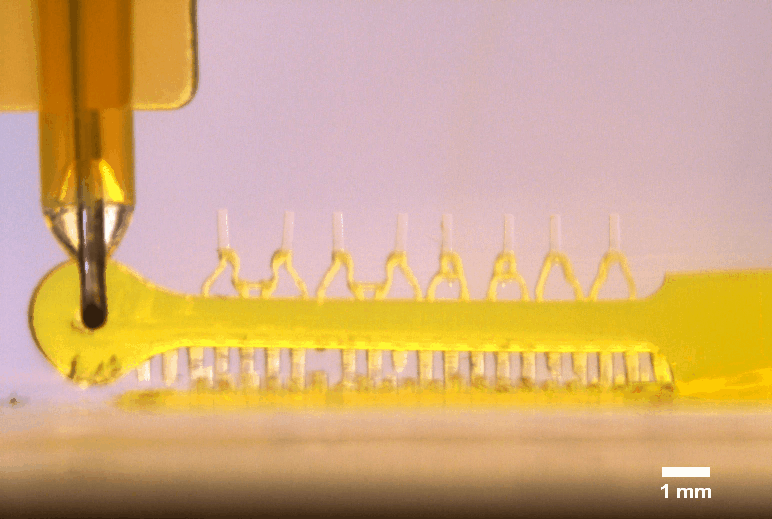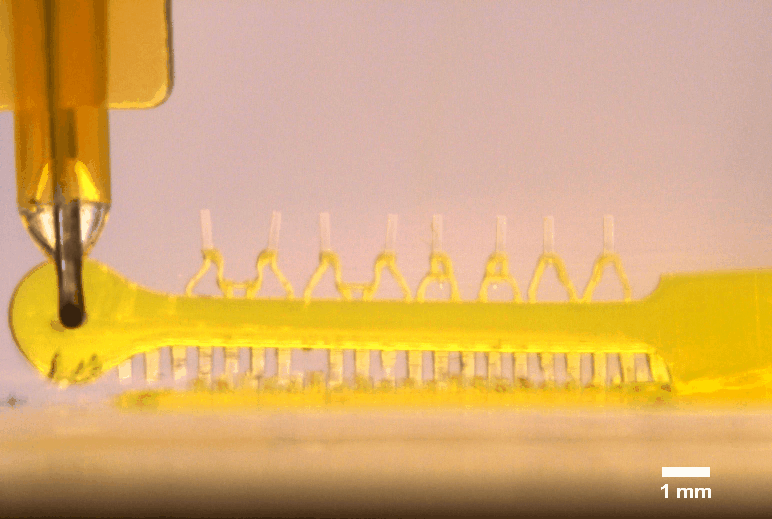
3D printed multi-microactuator with four pairs of microposts, enabling repeated parallel mechanical actuation of 4 tissue constructs at different strain levels to identify optimal mechanical stimulation conditions. The 3D printed design results from topology optimization in collaboration with colleagues at DTU Mechanical Engineering.
Group Leader
Niels Bent Larsen Group leader, Professor Department of Health Technology Phone: +45 45258161 nibl@dtu.dk

