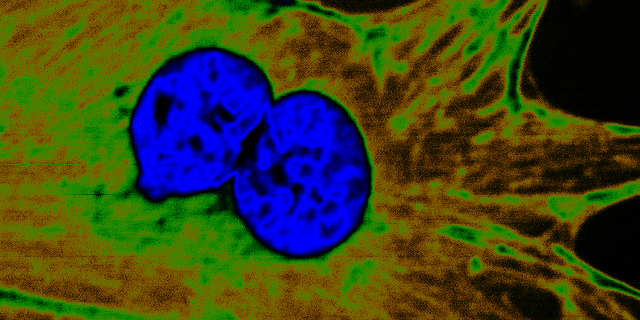
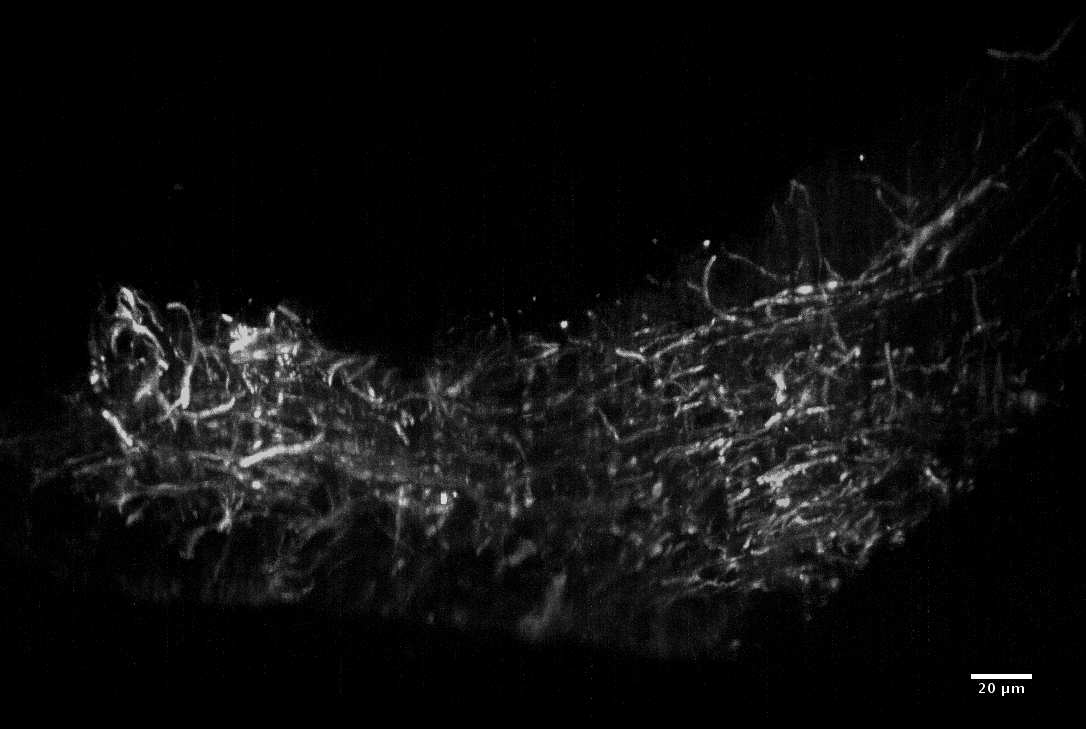
Optical imaging offers great promise for addressing unmet clinical needs due to the combination of non-invasive, real-time capture of biomedical information enabling point-of-care decisions. This enables earlier onset of treatment, reduced therapy costs, reduced recurrence rates, improved clinical outcomes, and a better patient experience. Moreover, in biology applications, optical imaging and nonlinear microscopy provide new opportunities for recording of metabolic and signalling information at the cellular level. Such understanding paves the way for new diagnostics, treatment, and drug development for a wide range of diseases.
Conventionally, optical imaging modalities are applied as standalone techniques each targeting one biomarker. However, recently it has been shown that diagnosis is significantly improved by combination of different contrast mechanisms in a multimodal approach. Hence, multimodal biomedical imaging, being considered the next generation technology within diagnostics, allows objective assessment of the status of disease, such as assisting staging and grading of cancerous lesions or tissue function monitoring. To transfer optical imaging technology into applications, systems would have to be compact, user-friendly, and fitted into endoscopes. Thus, photonic technologies and delivery systems for probing must be further developed.