The intestinal mucosa represents the largest surface of the body and is continually exposed to foreign material derived from our diet and the trillions of microorganisms residing within the intestinal lumen.
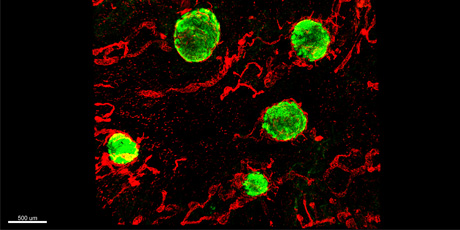
Figure legend: Intestinal lymphoid follicles in the human large intestine, identified by staining of CD45+ immune cells (green) and Lyve-1+ lymphatic endothelial cells (red).
Maintenance of intestinal homeostasis is dependent upon the immune system’s ability to remain tolerant to such material, while retaining the ability to mount appropriate immune responses to the many viral, parasitic and bacterial pathogens that utilize the intestine as a primary site of entry into the host. A breakdown in such mechanisms can result in intestinal pathology and is believed to contribute to the development and maintenance of inflammatory bowel diseases, including Crohn’s disease and ulcerative colitis, as well as food hypersensitivities such as celiac disease.
The laboratory focuses on understanding the mechanisms regulating intestinal immune responses in health and disease, with the long-term goal of identifying novel modalities for treatment of disease and more effective vaccine strategies. Our main focus is on the role of antigen presenting cells, in particular dendritic cells, as well as intestinal stromal cells in the regulation of mucosal immune responses.
Dendritic cells are innate immune cells that acquire antigens in peripheral tissues and then migrate to local tissue-draining lymphoid tissues, where they present self and foreign-derived antigens to cells of the adaptive immune system. We, and others, have recently identified several subtypes of intestinal dendritic cells, each with a unique ontogeny and function in the mucosal adaptive immune response.
Stromal cells are non-hematopoietic mesenchymal-derived cells that have primarily been implicated in tissue organization events. Our recent studies point to a marked complexity in mesenchymal stromal cell subset composition and function within and along the length of the intestine, and we are currently exploring the ontogeny of these subsets and their contribution to intestinal immune homeostasis and immune-mediated intestinal pathology.
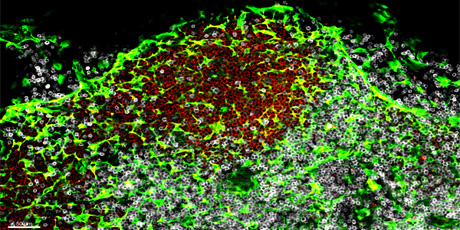
Figure legend: Transglutaminase-1+ mesenchyme-derived Fibroblastic reticular cells (green) in a Peyer’s patch in the human small intestine. The Peyer’s patch is organized into distinct B cell (identified by CD19+ cells in red) and T cell (identified by CD3+ cells in white) areas.
Our approaches combine the use of state-of-the-art transgenic animal models with cellular and molecular studies of human intestinal biopsies and re-section material. Key technologies include multi-parameter flow cytometry and imaging techniques, sequencing technologies (including single cell RNAseq), in vivo models of immune cell functionality, infection and inflammation, as well as in vitro co-culture techniques.
Press this link to see the full list of publications from the Agace group.